

Ionization energy is the energy required to remove an electron from a neutral atom or ion. In order to accomplish this, energy must be added to the gaseous atom.
Ionization involves the removal of electrons from the gaseous metal to form an ion. ½ Cl 2 (g) → Cl (g) ½ ΔH bond = +121 kJ/mol Step 2: Ionization Energy and Electron Affinity Each chlorine atom absorbs half of this bond energy. The chlorine molecule’s disassociation energy (ΔH bond) is 242 kJ/mol. Dissociation energy is the energy required to break apart a compound. Diatomic gaseous chlorine breaks into two individual atoms by absorbing energy.The sublimation energy (ΔH sub) of a solid sodium atom is 107 kJ/mol. Sublimation energy is the energy required to change the phase of an element from solid to gas. A solid sodium atom undergoes sublimation to form a gaseous atom by absorbing energy.Hence, energy is added to nonmetals to disassociate into atoms. On the other hand, nonmetals exist in polyatomic form.

Atomization is the formation of gaseous atoms from their naturally occurring elements. The elements involved in the reaction should be in their gaseous forms. Step 1: Sublimation Energy and Disassociation Energy Energy changes in each step except the lattice energy can be experimentally measured. The formation of ionic sodium chloride from solid sodium metal and gaseous chlorine is not a single-step process. The heat of formation (ΔH f 0), also known as enthalpy of formation, is 411 kJ/mole for sodium chloride. It also indicates that the process of formation is an exothermic one. Usually, this value is negative as ionic compounds are stable. The heat of formation is the enthalpy change when forming a compound from its elements. įormation involves the reaction between the elements in their standard states to form the ionic compound.
LATTICE ENERGY EQUATION BORN HABER CYCLE HOW TO
Let us understand how to use Born-Haber cycle to determine the lattice energy of sodium chloride (NaCl). It utilizes the known thermodynamic quantities like ionization energy and electron affinity. The Born-Haber cycle takes advantage of the enthalpy change to determine the lattice energy of ionic compounds indirectly.
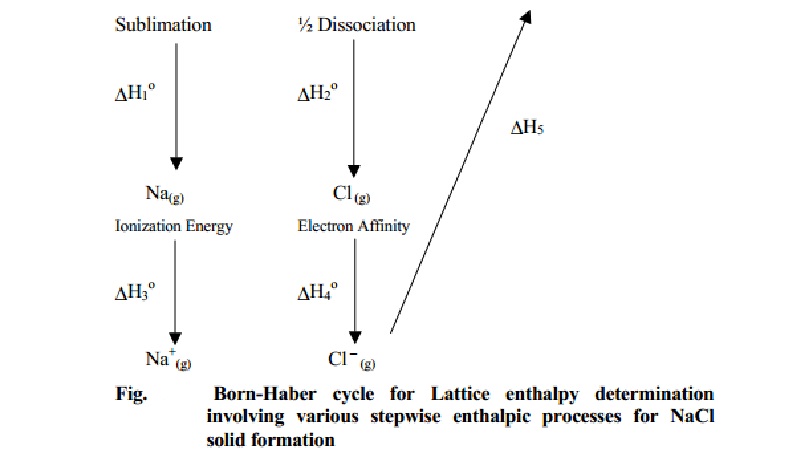
When summed over the individual steps, the total enthalpy change over a closed cycle is zero. Hess’ law states that the enthalpy change in a chemical process is independent of the path the process takes. The formation of a solid ionic compound from the elemental state of the constituent atoms goes through several steps and involves enthalpy in each step. īorn-Haber cycle uses Hess’s Law to find the lattice energy exclusively. It is due to the electrostatic force of attraction and is responsible for keeping the anions and cations of the compound in fixed positions. Lattice energy is the energy released when one mole of an ionic compound is formed from its constituents in their gaseous state. Born-Haber Cycle and Lattice Energyīorn-Haber cycle is used primarily to calculate lattice energy, which cannot typically be measured directly. The Born-Haber cycle is named after two German scientists, Max Born and Fritz Haber, who developed it in 1919. The process enables one to understand the overall reaction progress through steps by visually representing the energies involved in these steps. It is concerned with forming a stable ionic compound from reacting an alkali or alkaline earth metal with a nonmetal like a halogen or oxygen. Born-Haber cycle is a process that allows one to understand and analyze energies in a chemical reaction.


 0 kommentar(er)
0 kommentar(er)
